In a pharmaceutical organisation, Product Managers play a crucial role in establishing a product’s brand identity, in building awareness, and in maintaining credibility. They are uniquely positioned to understand and scope out market trends, to develop the product’s brand strategic direction, and to coordinate and communicate marketing plans.
As the gatekeepers of product brand marketing, Product Managers manage existing product lines and build launch strategies for new products. Through their work with Key Opinion Leaders and their deep insight into brand building, Product Managers gain a keen sense of what the medical community wants and needs, and how the community views current healthcare priorities, challenges and opportunities.
A well-researched, engaging and timely marketing plan is the backbone of a successful pharma marketing campaign. When it comes to building a product’s brand presence in multiple international target markets, the key to success lies in marrying the campaign with skilled localisation, i.e., collaborating with the right localisation partner.
In international markets, a new product stands or falls on the merit of its local reach, credibility and engagement.
In this blog, we explore three key elements of pharma marketing through the eyes of a Product Manager running a successful localisation campaign: product brand credibility, brand engagement and brand reach.
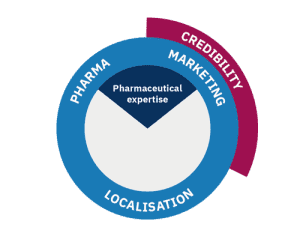
Credibility
Healthcare professionals and their patients share one overarching concern – the drugs and medicines they use must be safe and effective. Trust plays a hugely important role in marketing a new drug and in maintaining a brand’s existing market presence.
As such, a product’s brand credibility is one of the cornerstones of successful pharmaceutical marketing. In international markets, that credibility is communicated through translation.
In pharmaceutical translation, credibility rests in the hands of professional, domain-expert translators. They understand the science behind the words and use the right medical or lay terminology, depending on the target audience.
This is the first key element of pharma marketing localisation – building product brand credibility through translation by expert pharmaceutical translators.
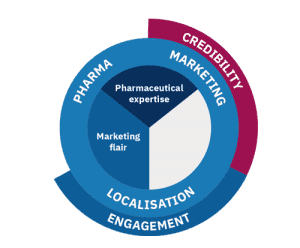
Engagement
Pharma marketing might focus on pharmaceutical products, but it’s still marketing. Its key aim is to build brand awareness and engagement, and to sell a product. Pharma marketing takes a sophisticated, rigorously tested product and introduces it to the world in an engaging, effective manner.
A clever pharmaceutical marketing plan identifies the need to review and, where necessary, adjust brand messaging for local markets. The witty punchline that works so well in English won’t necessarily translate into French, and might even be offensive if converted directly into Finnish.
Marketing translation is an art in itself. A marketing translator with flair doesn’t just translate the content, they transcreate it to convey the message behind the words. Their goal is to create engagement and match the impact of the source text – but in a completely different target culture and language.
This is the second key element of pharma marketing localisation: creating engagement using the right marketing touch and flair.
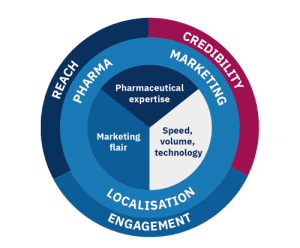
Reach
But credibility and engagement alone don’t make a successful pharma marketing campaign. The message also needs to reach its audience in the right place, at the right time, over and over again.
In building reach into the marketing campaign, the goal of a Product Manager is to create a versatile suite of marketing materials for a number of different purposes, in different formats. All these materials must then move into the localisation phase, of course, but by the time localisation happens in the lifecycle of a marketing campaign, it’s usually already urgent.
Within a pharma marketing campaign, the role of localisation is not just to deliver professional, engaging, high-quality marketing translation. It’s critical that localisation is delivered at speed, and in volume, using the right technology.
This is the third key element of pharma marketing localisation: choosing a professional team who can deliver in volume, at speed and using the right technology.
Building a Brand: What Good Localisation Looks Like
Product Managers at a pharmaceutical company have to keep lots of plates spinning at once. They build, develop and maintain brand awareness and engagement of multiple products across several digital and traditional touchpoints. The strategies and campaigns they design can make or break a product in a local market.
A good localisation service provider understands the challenges of pharma marketing and the need for combining pharmaceutical expertise with marketing flair.
In pharmaceutical translation, trust is built on credibility. Credibility in translated marketing content is built by a savvy Product Manager choosing the right localisation partner with the right expert resources.
Engagement is fostered through compelling marketing copy. In international markets, a switched-on Product Manager knows that the role of localised copy is to compel and engage, and ensures that localisation teams have the right marketing expertise.
Reach is about delivering your message to the target audience at the right time and place so that the brand’s credibility can shine through, thus equating to engagement. A Product Manager running an international marketing campaign ensures that their localisation partner has the right resources – both human and technological – to deliver in volume, at speed and with the right technology, without compromising quality.
For your localisation service provider, this means that:
- Account Managers build localisation for the dual pharma-marketing aspect.
- Project management workflows are agile and responsive.
- Translators are vetted, tested, subject-matter experts, with proven marketing flair.
- Translation teams are sufficiently large to handle content in volume and at speed, without compromising quality.
- Translation consistency and terminology is managed through sophisticated language technology solutions.
- Documentation is double-checked and formatted correctly at delivery.
- Deliveries are correct, timely and complete.